
Eligibility
Local study conflicts
- None
Inclusion Criteria:
- Aged 16 years old or over
- A current clinical diagnosis of cellulitis at any body site except the orbit (periorbital/orbital cellulitis)
- Able to provide informed consent
- People of child-bearing potential must be willing to:
- Use an effective method of contraception** (and must agree to continue 3 months after the last dose of the IMP)
- Inform the trial team if pregnancy occurs during trial participation
Exclusion Criteria:
- Orbital or periorbital cellulitis, surgical site infection, or planned surgical management (e.g abscess) as managed under a different clinical pathway
- Allergy to dexamethasone
- Contraindication to dexamethasone due to concurrent medication (e.g. cobicistat)
- Has known current invasive fungal infection
- Has known current gastric or duodenal ulceration
- Already on corticosteroids
- Unable to take oral medication
- Lack of capacity
- Inability to complete follow-up procedures
- Prisoner
- Pregnant, breastfeeding, or planning to conceive in next 3 months
If the patient is eligible:
Gain verbal agreement in principle before proceeding. Explain:
- Taking part in research is likely to improve the quality of their care
- Risks are carefully controlled - research is safe
- Explain the uncertainty that exists with respect to treatment of their condition;
- If they change their mind they can withdraw their participation at any point
Step-by-Step Guide to Recruitment:
In the working hours of the research team:
- Monday-Friday 08:00 – 16:00
- Contact Muni on ext 86981 or 86653
or message Wuraola Akande via Teams chat
Out of hours and weekends:
Estimated time required to recruit a patient:
20 minutes
Gather documentation
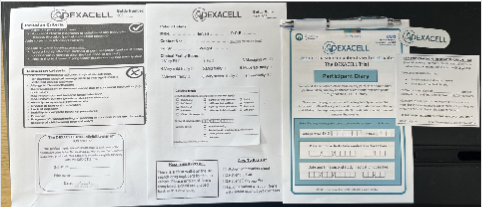
- Paper:
- Grab packs contain all necessary documents are in the Research Drug Cupboard in the OU medication room (next to sulce)
- Inside each pack: Participant Information Sheet, Consent Form, Eligibility form, Pre-Randomisation form, Intervention 1st dose form, Patient ID Card & Patient Diary.

- Online:
- Play the patient the participant information video found here: https://youtu.be/bNMIS_ozuu4
- The diabetes information sheet and general PIS can be found at the bottom of the page.
- Informed consent form must be accessed via RedCAP, if you do not have RedCAP access please use paper documents.
Confirming eligibility
- Eligibility can only be confirmed by doctors who are on the delegation log and have a GCP certificate.
- If you are not on the delegation log (+/- GCP) you can find a names of delegated staff on the ED Open Research Teams page Here (opens in a new tab)
- Once you have confirmed eligibility please COMPLETE AND SIGN The ELIGIBILITY SCREENING FORM
Consenting the patient formally:
REMEMBER you must be on the dexacell delegation log, have a current GCP certificate and have completed the trial training in order to consent a patient
- Consent recorded on PAPER:
- After the participant has had sufficient time to review the printed PIS: provide the paper consent form to the patient for review and completion, remaining available to answer any queries they may have.
- Check the patient has completed the form correctly and countersign the form as the staff member receiving consent.
- Add the participant ID to the consent form (ID generated when adding record to the REDCap, if you don't have REDCap access please contact Muni on 86981 or via the EPICS out of hours)
- Take copies of the consent form and give a copy to the patient, and place the original consent form in the Grab pack envelope (Research team will upload to REDCap and eCare)
- Document in medical notes on eCare 'Patient approached and consented to particpate in DEXACELL Trial. Written consent obtained from patient. Study ID: DX_ _ _ _ _ _ _. Bottle No:_ _ _ _ _ _ _ _.
- Consent recorded ELECTRONICALLY:
- Complete the ‘Pre-Consent’ eCRF indicating electronic consent will be used. You must provide the version and date for the PIS given. After the participant has had sufficient time to review the printed PIS: open the ‘Consent’ eCRF and click the link to the electronic consent form
- Pass the device to the patient to review the consent form and enter initials into statement boxes and add their signature. Guide the patient through the form and help with navigation if required.
- Review the form for correctness and completeness, ensuring a proper signature has been given, then countersign electronically as the staff member receiving consent and click ‘Next Page’.
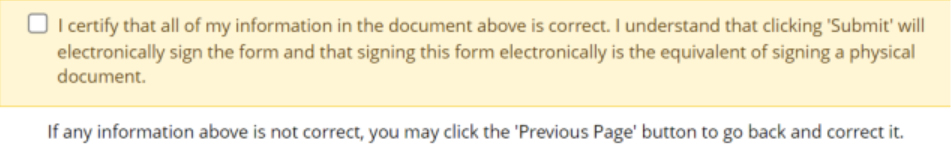
- A window with the completed form will show for review. A copy of the form can be downloaded here by clicking the save button. A pop-up will appear to confirm name and save location.
- If all information in the form is correct, tick the box to certify, and click submit to finalise the form.
- Consent provided VERBALLY:
- If the patient has the capacity to consent verbally but cannot physically sign, proceed with obtaining verbal consent for each point on the consent form in the presence of an independent witness (e.g. a member of hospital staff outside of the trial team).
- If possible, encourage the patient to mark the signature space on the form in the best way they can, though this is not essential if not possible and if the witness signs the form.
- The witness must Initial the statement boxes on the consent form after each statement is verbally agreed upon by the patient.
- They should use their own initials, and not the patient’s.
-Sign their name in the witness box on the consent form
Randomisation procedure
You must have REDCap access to randomise.
If you do not have access please contact Muni via 86981 or Wuraola Akande on Teams. Alternatively you can check the delegation log
herefor a list of ED staff with access.
- Log into [DEXACELL REDCap Link HERE]
- Go to the Randomisation eCRF
- You will be prompted to ensure there is enough stock to randomise
- If there is adequate stock, enter your name and role.
- Another question will appear: ‘Can randomisation go ahead?’ Select ‘yes’ if there is sufficient stock and patient is able to receive first dose of medication immediately.
- Save the form - this will trigger randomisation. Please only save the randomisation form once, clicking save a second time may cause an error.
- The form will automatically lock once complete. If the site staff need to unlock the randomisation form for any reason, please email: dexacell@exeter.ac.uk or call: 01392 726 337.
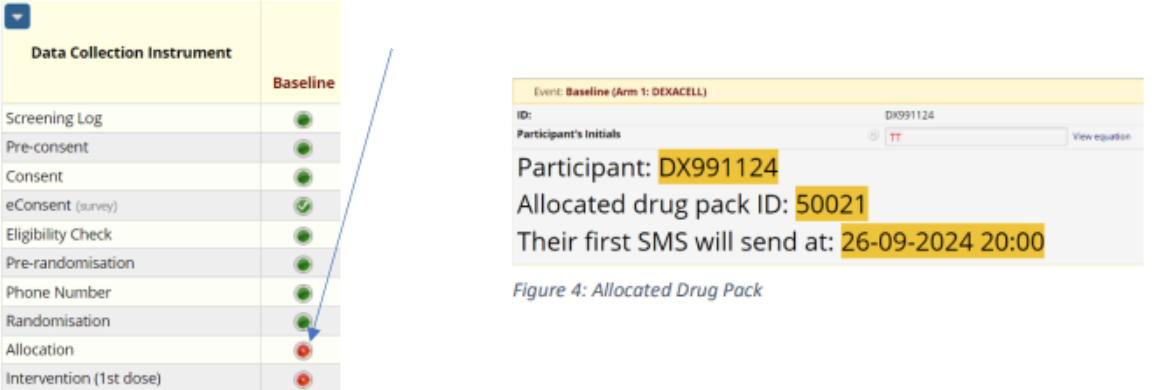
- The system will return you to the participant’s record. Under the column ‘Baseline’, the ‘Allocation’ eCRF will display as red.
- Click on the red circle to open the Allocation CRF.
- The participant’s allocated drug pack ID will display like this:
- Saving the Allocation eCRF will display the status as green
Back-up randomisation process
- If you have forgotten your log in details, please email DEXACELL@exeter.ac.uk or call 01392726337. The CTU team will send a reset password link from to your email address.
- If you are unable to access REDCap to randomise due to a system outage (e.g. internet failure) at site, you can call Exeter Clinical Trials Unit on 01392 726 337 who can randomise on your behalf. This service is only available Monday – Friday 9am – 5pm, and will not be available on weekends, bank holidays or University of Exeter closure days. If you have technical problems, outside of these hours there is unfortunately no other back-up randomisation process due to the blinded nature of the intervention and the mechanics of our IMP management system. If calling ExeCTU for back-up randomisation - please have the data required for the eligibility check, pre-randomisation and phone number eCRFs available to relay over the phone (unless you have already entered this data on REDCap). You can use the source data worksheets to collect this data if required
Carrying out the intervention:
- The DEXACELL IMP needs to be prescribed on eCARE, as you would with any other medication in the trust. But, do not prescribe dexamethasone!
- Click on Request/Care Plans
- Click Add
- In the search bar type in DEXACELL
- On the next page select Inpatient (If the patient is likely to be admitted into hospital) or Discharge (if you expect them to go home from A&E), then click sign
- On the next screen add participant ID & Drug pack ID to the relevant fields/ comment box- Do not miss this out. Then click sign to generate the prescription.
Prescribing:
- Ideally done by 2 people
- Get the Research drug key from NIC/EPIC Go to the Research drug cupboard (in OU, next to sluice)
- DEXACELL IMP are on the top shelf, take the pack that corresponds with the randomisation details only (Allocated drug pack ID should match randomisation details and eCARE prescription) If the matching pack is not available or not in the cupboard please immediately contact the Research team &/or Pharmacy 07971124621. Do not proceed any further.
Dispensing:
- A full check must be undertaken by two competent members of staff to confirm that the drug pack allocation and participant details on the randomisation system matches the details of the participant being prescribed to and the pack ID selected for dispensing. This check should be recorded on the prescription.
- DOSE 1 (2 CAPSULES)-Dose 1 should be administered after randomisation.
- Participants should be counselled about how the dexamethasone/placebo should be taken, the dosage schedule and the potential side effects.
- Dose 1 (2 capsules) should then be taken in the presence of the prescribing & dispensing staff member.
- The time that Dose 1 is taken should be recorded on the ‘Intervention (1st dose)’ eCRF on REDCap and on the participant’s diary.
- The time that Dose 2 is due (24 hours after Dose 1) should also be recorded on the participant’s diary and on their drug chart if they are an inpatient.
Administration:
Completed documents
- Please place paper consent form back in envelope, complete both sides of the envelope and place envelope back in clear wallet on the drug cupboard door
Disposition and follow-up:
DOSE 2 (2 CAPSULES) should be stored securely in the original packaging
If admitted:
- Inpatient: If the participant is still hospitalised or waiting to be admitted when they are due Dose 2 be handover handed over to the clinical team with the DEXACELL handover document. This will outline basic relevant details of the trial and details of the study drug including what it may contain (active drug/placebo) plus the time and method of administration for Dose 2.
- It will instruct the person administering the IMP to record the administration in the medical notes and to communicate clearly to the participant that they are being given the second dose of the study drug.
- Participants will be asked to confirm that they have taken this second dose at the 3rd follow up timepoint (T3).
If discharged:
- Discharge patient - If the participant is discharged from hospital before Dose 2, the participant should be given Dose 2 (2 capsules) in the bottle to take home with them.
- They should be given clear instructions on when to take Dose 2 (as close to 24 hours after Dose 1 as possible, +/- 6 hours).
- The time Dose 2 is due should have been noted on the participant’s diary which they will take home with them. It may be useful to suggest setting an alarm or an alternative reminder at the time Dose 2 is due.
- Remind Participants will be asked to confirm that they have taken this dose at the 3rd follow up timepoint (T3).
Unblinding procedures
- A trial-specific procedure is in place for emergency unblinding although with both the short-term intervention period and low risk of serious adverse events it is anticipated that the need for unblinding will be minimal.
- Emergency unblinding will be available 24/7 via a dedicated and automated phoneline. Further details will be provided in a separate unblinding work instruction.
The procedure for emergency unblinding can be found in the blue research folder in the doctors room in A&E
Links for further information:
Tap these buttons for QR code links to the patient information video and text sheets, The link for the study homepage is at the foot.


