
Eligibility
Local study conflicts
- None:
Co-enrolment with observational studies is permitted without prior agreement
Inclusion Criteria:
- Hospitalised infant aged <12 months with a clinical diagnosis of acute bronchiolitis
AND - Clinically assessed at least twice, 15 minutes apart, to have either:
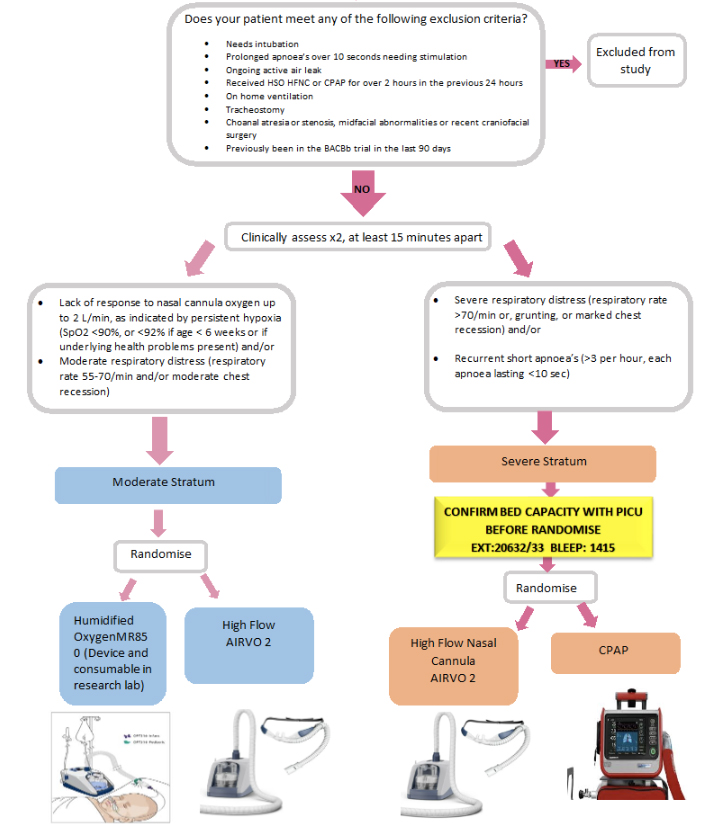
- A. SEVERE BRONCHIOLITIS arm of the study:
Severe respiratory distress (respiratory rate >70/min, or grunting, or marked chest recession) and/or recurrent short apnoeas (>3 per hour, each apnoea lasting >10 sec)
OR- B. MODERATE BRONCHIOLITIS arm of the study:
Lack of response to LFNC oxygen up to 2 L/min as indicated by persistent hypoxaemia (SpO2 <90%, or <92% if age < 6 weeks or if underlying health problems present) - and/or
- Lack of response to LFNC oxygen up to 2 L/min as indicated by moderate respiratory distress (respiratory rate 55-70/min and/or moderate chest recession)
- A. SEVERE BRONCHIOLITIS arm of the study:
Exclusion Criteria:
- Clinical decision that patient needs immediate intubation and ventilation for life-threatening hypoxaemia, shock or decreased conscious level.
- Prolonged apnoeas (>10 seconds needing stimulation).
- Ongoing active air leak (pneumothorax, pneumomediastinum).
- Received HSO, HFNC or CPAP for over 2 hours in the previous 24 hours.
- On home ventilation prior to hospital admission.
- Tracheostomy in place.
- Choanal atresia/stenosis, midfacial anomalies or recent craniofacial surgery.
- Previously recruited to the BACHb trial in the last 90 days
If the patient is eligible:
- Gain verbal agreement in principle before proceeding. Explain:
- Taking part in research is likely to improve the quality of their care
- Risks are carefully controlled - research is safe
- Explain the uncertainty that exists with respect to treatment of their condition
- If they change their mind they can withdraw their participation at any point
- The study utilises a deferred consent model (‘research without prior consent’, RWPC) in the BACHb trial. Written informed consent will be obtained from parents/legal guardians as soon as practically possible after randomisation
Step-by-Step Guide to Recruitment:
In the working hours of the research team:
- Monday-Friday 07:30 – 18:30
- Contact the EMROx research team on:
Mobile 0776818242
Tel 01865 222003
Bleep 6680
Out of hours, weekends, and when the research nurse is not available:
Estimated time required to enroll
- 20-30 minutes
First; stratify the patient into the appropriate group using the recruitment pathway diagram:
Patient recruitment pathway:
Click for a larger image
Gather documentation
- Documentation is stored in the research drawer in the Paediatrics ED, including:
Study protocol
Recruitment guide
Treatment flowchart
Randomisation cards
Participant information sheet (PIS)
Consent form

Gaining formal consent
- The study utilises a deferred consent model (‘research without prior consent’, RWPC) in the BACHb trial. Written informed consent will be obtained from parents/legal guardians as soon as practically possible after randomisation
Randomisation procedure
- For patients with severe bronchiolitis: Do not randomise if there is no an available bed in PICU.
- Eligible patients will be randomised using the Sealed Envelope website here. (https://www.sealedenvelope.com/access/apps.) Staff who are on the delegation log have individual access.
Carrying out the intervention:
- SEVERE arm of the study
- CPAP will be started in PICU. Whilst the patient is in ED the patient will receive standard care
- HFNC AIRVO2 can be started in the ED
- MODERATE arm of the study
- HSO (Humified Oxygen) – MR850 device and consumables are in the research Lab
- It or HFNC via AIRVO2 will be started in ED
- Please see the further Guidance notes; available here or click the image below

Disposition and follow-up:
- All patients enrolled in this study will be admitted from the ED, (due to the severity of their bronchiolitis.)
- As all participants are in-patients then they will be continuously monitored by the clinical care team and any incidental findings will be identified by the clinical care team and acted on accordingly as per routine care.

